It is evident that tooth loss alters both the structural and functional relationships of the patient’s jaw and it also has a cosmetic effect when patients lose teeth. The loss of a tooth affects the surrounding tissue, which no longer has to supply a tooth root with blood and oxygen, and as a consequence, there is a loss of bone substance in the surrounding tissue.
Endodontic treatment is performed by general dentists and specialists when the pulp is damaged in one way or another, as a result of infection or trauma. It can be direct trauma or indirect consequences of damage to the periradicular tissue.
Endodontic treatment can be surgical or non-surgical.
1.
To clean the root canal so that it is as clean as possible without bacterial intrusion.
2.
To seal the root canal at the apex so that there is no ingress of fluid from the surrounding tissue and no material from the root seal leaking into the periapical tissue.
3.
Fill the root canal with a suitable and tested root filling material.
4.
Make a coronal seal that protects the root canal from being contaminated with saliva and microorganisms from the oral cavity.
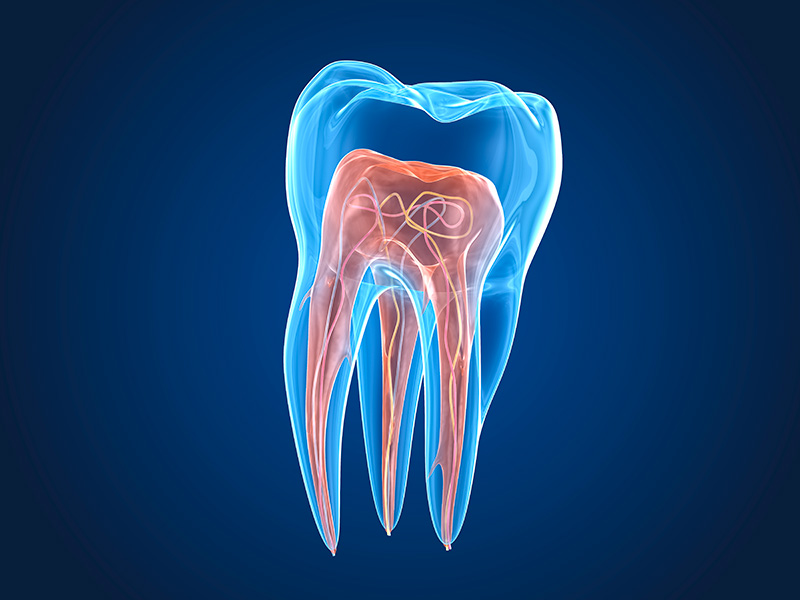
The criterion for achieving a satisfactory endodontic treatment is to obtain a clean root canal free of tissue debris and bacteria so that the root canal can be shaped and prepared to be filled with a suitable root filling material. The root filling must meet criteria that provide a tight seal at the apex, a tight filling of the root canal, and a tight seal coronally.
Read more about our two root filling systems SoftCore® and OneStep®
1.
The root canal is not sufficiently cleaned and tissue residues and/or microorganisms remain in the canal.
2.
The root canal is not sufficiently sealed to allow microorganisms to enter.
3.
The root filling material does not fill the canal sufficiently against canal walls and inside canals.
4.
Root canals have been overlooked and not filled.
5.
That there is excess root filling material that has penetrated the periapical tissue.
One of the main causes of a failed root canal treatment is the presence of microorganisms in the root canal. The role of bacteria in periradicular infection is well documented in the literature and it is well known that there is an increased risk of a secondary infection in the root canal if bacteria are present in the lateral canals when the tooth is rooted. It is almost impossible to eliminate all bacteria in the isthmus and dentin tubules with disinfectants alone, as the products cannot penetrate everywhere.
Soft-Core® Endodontic Obturator
One-Step Obturator™
All development and production of the two patented root filling products have been done in collaboration with the Danish Technological Institute. CMS Dental specializes in the manufacture of precision root canal filling products. Everything is produced in our factory in Glyngøre.
The story begins with Soft-Core, which was developed and marketed in 1996, and later One-Step was added.

For more than 25 years CMS Dental has been producing dental obturators.
CMS Dental is the only company in the world to use natural Gutta Percha for its root canal filling products.
Natural Gutta Percha is the raw material approved for use in root canal filling of teeth and it has proven to be the optimal material for root canal filling.
Natur Gutta Percha has been used for more than 100 years for root canal filling of teeth.
Every year, 1 million root fillings are performed with Soft-Core worldwide.
Before you root fill a tooth, you need to make sure that the root canal is free of bacteria.
CMS Dental has therefore developed a treatment concept to eliminate bacteria in the root canal, called LAD (Light-activated disinfection), and consists of a light source and a photosensitizer. The light activates the photosensitizer, which produces reactive oxygen that kills microbes in the root canal in milliseconds. Where light can penetrate and activate the photosensitizer, the method is highly effective and without side effects.